Dihydroxylation using osmium tetroxide progresses with syn stereochemistry. Oxidation and reduction of alkenes and alkynes.
Alkane To Alkene Oxidation Or Reduction. Oxidation, oxidative cleavage, and reduction reactions for alkenes and alkynes.need help with orgo? Alkenes undergo a number of reactions in which the c=c double bond is oxidized.
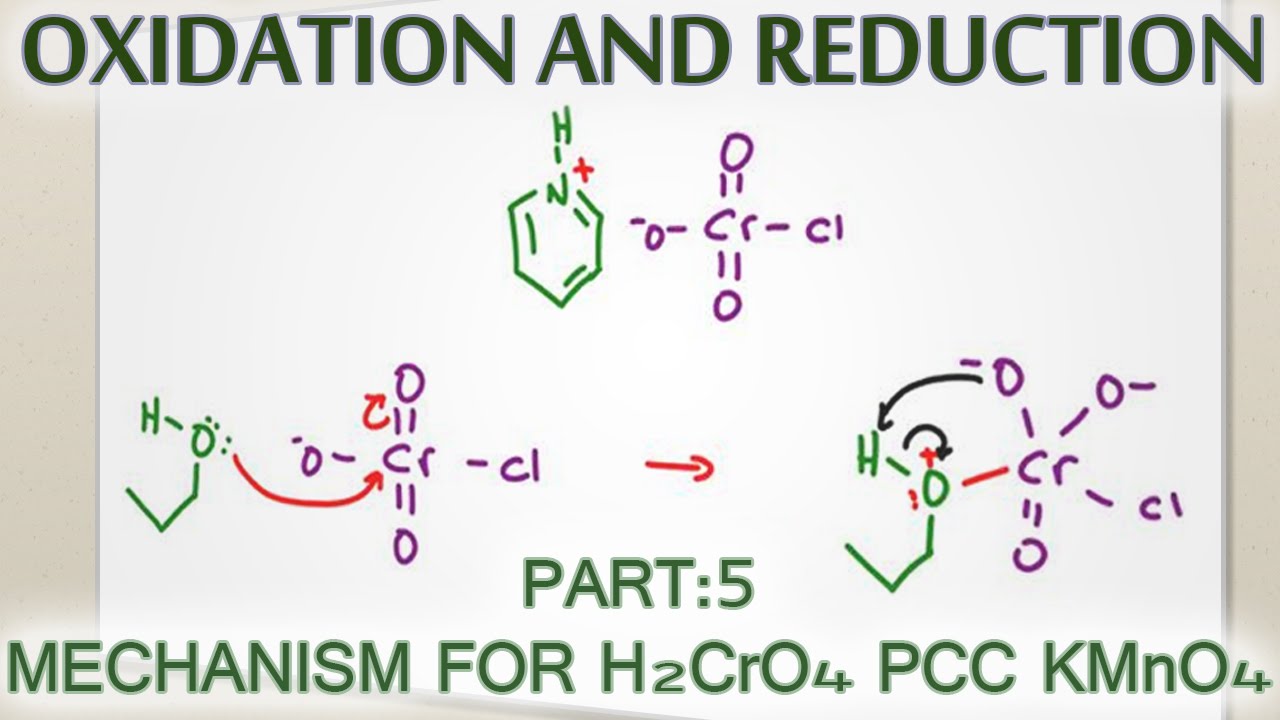
 Alcohol Oxidation Mechanism with H2CrO4, PCC and KMnO4 From youtube.com
Alcohol Oxidation Mechanism with H2CrO4, PCC and KMnO4 From youtube.com
14.6 reduction of alkynes a. This reaction of alkenes happens on the surface of a metal catalyst. Reduces ketone or aldehyde into alkane zn(hg)/hcl.
Alcohol Oxidation Mechanism with H2CrO4, PCC and KMnO4
• the reaction uses h2 and a precious metal catalyst. Oxidation, oxidative cleavage, and reduction reactions for alkenes and alkynes.need help with orgo? The selective oxidation of hydrocarbons is a challenging reaction for synthetic chemists, but common in nature. Hydrogenation of a double bond is a thermodynamically favorable reaction because it forms a more stable (lower energy) product.
 Source: psu.pb.unizin.org
Source: psu.pb.unizin.org
In this video we differentiate between redox reactions vs reactions that add oxygen/hydrogen without a net reduction. It involves the addition of two hydroxyl groups across the double bond with two different stereochemical approaches, namely anti and syn. The typical catalysts for the alkene hydrogenation. Alkenes can easily be oxidized by potassium permanganate and other oxidizing agents. 2.) the solvent.
 Source: youtube.com
Source: youtube.com
Hydrogenation of a double bond is a thermodynamically favorable reaction because it forms a more stable (lower energy) product. Describes the application of the definitions of oxidation and reduction to the general transformations of alkanes, alkenes, alkynes and aromatic hydrocarbons. In a hydrogenation reaction, two hydrogen atoms are added across the double bond of an alkene, resulting in a saturated.





