
Many of these molecules are used in the production of other materials, such as plastics, but. 10.1 synthesis of alkenes 10.1.1 dehydrohalogenation of alkyl halide.
Alkane To Alkene. In an alkane, all 4 4 4 valencies of the carbon atom are satisfied with other hydrogen atoms. Alkanes, alkenes, and alkynes are all organic hydrocarbons.
 Fettsäuren, Schmelzpunktet From u-helmich.de
Fettsäuren, Schmelzpunktet From u-helmich.de
In an alkane, all 4 4 4 valencies of the carbon atom are satisfied with other hydrogen atoms. Many of these molecules are used in the production of other materials, such as plastics, but. Unsaturated hydrocarbons such as alkenes and alkynes are much more reactive than the parent alkanes.
Fettsäuren, Schmelzpunktet
Although there are similarities between alkanes and alkenes such as nonpolar behavior and insolubility in water, they have many distinct features. 10.7.2 oxidative cleavage of alkenes. Alkanes and alkenes written by tutor nathan r. An organic molecule is one in which there is at least one atom of carbon, while a hydrocarbon is a molecule which only contain the atoms hydrogen and carbon.
 Source: youtube.com
Source: youtube.com
Start studying alkanes and alkenes. Alkanes, alkenes, and alkynes are all organic hydrocarbons. Chemical tests for alkanes, alkenes, and aromatic compounds, page 2 positive test for the presence of a double bond. This means that they have similar chemical properties to each other and they have trends in physical properties. Compound composed of only carbon and hydrogen saturated hydrocarbons :
 Source: slideshare.net
Source: slideshare.net
Although this is a good test to differentiate alkanes and aromatics from alkenes, other materials besides alkenes react with aqueous kmno 4. Dealing with organic compounds in chemistry can feel overwhelming. Compound composed of only carbon and hydrogen saturated hydrocarbons : Alkanes, alkenes, and alkynes are all organic hydrocarbons. Alkene + h 2 so 4 alkyl hydrogen sulfate
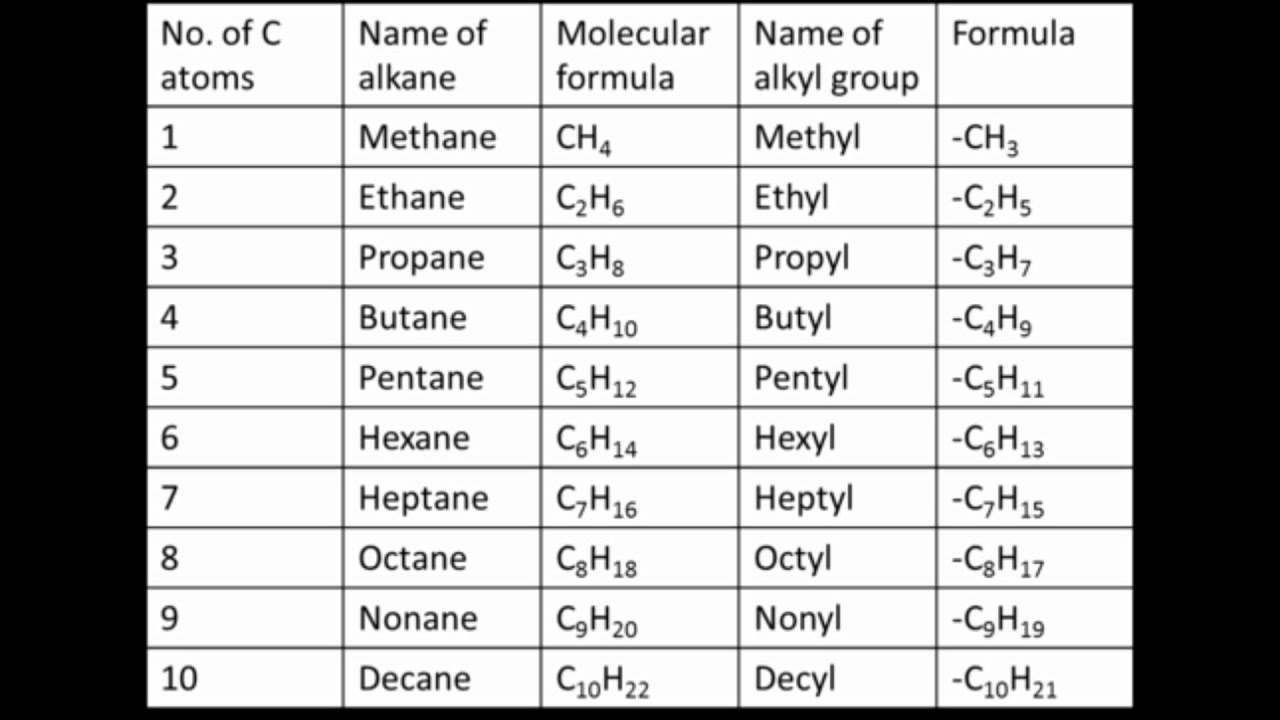 Source: youtube.com
Source: youtube.com
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds. Alkanes are solid, liquid or gas at room temperature depending on the size of their molecules.to learn detailed structures, formulas, and physical properties of alkanes with faqs and videos, visit byju’s for more information. Orientation of addition if hcl adds to an.
 Source: youtube.com
Source: youtube.com
Many of these molecules are used in the production of other materials, such as plastics, but. Although this is a good test to differentiate alkanes and aromatics from alkenes, other materials besides alkenes react with aqueous kmno 4. Alkene + h 2 so 4 alkyl hydrogen sulfate This holds true for the two compound groups, alkanes and alkenes. Although there.
 Source: youtube.com
Source: youtube.com
With stronger oxidizing agent being applied, the c=c double bond of alkenes can be oxidatively cleaved, and the alkene molecule is cleaved to smaller molecules. This reaction provides a way to test for alkenes or alkynes. Chirik and colleagues also found that light response can turn on both incurable and cyclic alkenes, while heat reaction leaves cyclic alkene untouched. However,.
 Source: youtube.com
Source: youtube.com
What type of reaction is alkene to alkane? To convert an alkane to an alkene, requires that you remove hydrogen from the alkane molecule at extremely high temperatures. Alkanes are solid, liquid or gas at room temperature depending on the size of their molecules.to learn detailed structures, formulas, and physical properties of alkanes with faqs and videos, visit byju’s for.
 Source: slideserve.com
Source: slideserve.com
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds. This process is known as dehydrogenation. Figure 10.1a e2 elimination of alkyl halide to synthesize alkene. Alkene + hydrogen → alkane this is called hydrogenation , and it needs a catalyst. Alkenes can be straight chain, branched chain, or cyclic.
 Source: slideserve.com
Source: slideserve.com
Figure 10.1a e2 elimination of alkyl halide to synthesize alkene. Many of these molecules are used in the production of other materials, such as plastics, but. However, the iupac recommends using the name alkene. To convert an alkane to an alkene, requires that you remove hydrogen from the alkane molecule at extremely high temperatures. With stronger oxidizing agent being applied,.
 Source: u-helmich.de
Source: u-helmich.de
However, the iupac recommends using the name alkene. Chirik and colleagues also found that light response can turn on both incurable and cyclic alkenes, while heat reaction leaves cyclic alkene untouched. Compound with only single bonds unsaturated hydrocarbons : C x n h 2 n + 2. Difference whatsoever between these alkenes and the symmetrical ones described above.





