
Alkane, alkene, alkyne & aromatic learning objectives 1. Also referred to as paraffin.
Alkane Alkene Alkyne Aromatic. (c) according to the resonance model of bonding, benzene is best described as a hybrid of two equivalent contributing structures. Alkynes are unsaturated carbon that shares a double bond at the carbon site.
 Oc 02functionalgroupshandout12087049304801899 (1) From slideshare.net
Oc 02functionalgroupshandout12087049304801899 (1) From slideshare.net
By reason of their formula alkanes are said to have no degrees of unsaturation. Unsaturated hydrocarbons unsaturated hydrocarbons § have fewer hydrogen atoms attached to the carbon chain than do alkanes § are alkenes with double c=c bonds § are alkynes with triple c≡c. The electrons that might be fixed in three double bonds are instead delocalized over all six carbon atoms.
Oc 02functionalgroupshandout12087049304801899 (1)
Chapter 12 alkenes, alkynes, and aromatic compounds 12. Due to resonance structures, the aromatic ring is extremely stable and does not undergo the typical reactions expected of alkenes. Alkenes, alkynes, and aromatic hydrocarbons are called unsaturated hydrocarbons they contain less hydrogen than an alkane having the same number of carbon atoms hydrocarbons alkenes: (c) according to the resonance model of bonding, benzene is best described as a hybrid of two equivalent contributing structures.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Hydrocarbons that contain a c bond h c c h (ethyne) Polynuclear aromatic hydrocarbons ( pahs) is another large group of hydrocarbons materials with two or more fused aromatic rings. C x n h 2 n + 2. Unsaturated hydrocarbons unsaturated hydrocarbons § have fewer hydrogen atoms attached to the carbon chain than do alkanes § are alkenes with double.
 Source: study.com
Source: study.com
4.1 alkanes, alkenes, alkynes and aromatic hydrocarbons. • molecules with the same functional group tend to Alkene + h 2 so 4 alkyl hydrogen sulfate note: Alkynes are unsaturated carbon that shares a triple bond at the carbon site. Alkenes have the general formula c n h 2n, and are examples of unsaturated hydrocarbons.
 Source: slideserve.com
Source: slideserve.com
(c) according to the resonance model of bonding, benzene is best described as a hybrid of two equivalent contributing structures. C x n h 2 n + 2. Alkenes, alkynes, and aromatic compounds alkenes and alkynes unsaturated contain bond to added. (or alkyne) like double bonds. Find the longest chain containing the double (alkene) or triple bond (alkyne) name the.
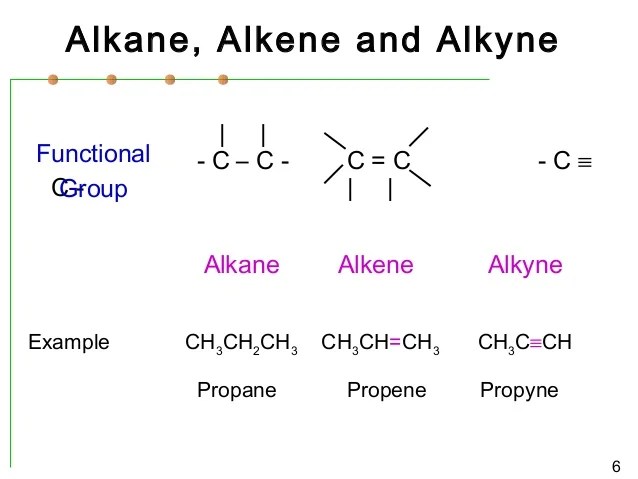 Source: slideshare.net
Source: slideshare.net
1 alkenes and alkynes general, organic, and biological chemistry copyright © 2010 pearson education, inc. Alkanes and aromatics from alkenes, other materials besides alkenes react with aqueous kmno 4. The presence of a double bond compound�s structure causes there. The electrons that might be fixed in three double bonds are instead delocalized over all six carbon atoms. Find the longest.
 Source: slideserve.com
Source: slideserve.com
(c) according to the resonance model of bonding, benzene is best described as a hybrid of two equivalent contributing structures. The electrons that might be fixed in three double bonds are instead delocalized over all six carbon atoms. Alkene + h 2 so 4 alkyl hydrogen sulfate note: Methane gas is the first member of the homologous series of alkanes..
 Source: slideserve.com
Source: slideserve.com
(d) benzene is a planar molecule. C x n h 2 n + 2. Hydrocarbons that contain a c = c double bond h2c = ch2 (ethene) alkynes: Hydrocarbons that contain a c bond h c c h (ethyne) Based on the molecular formula, determine whether each compound is an alkane, alkene, or alkyne.





