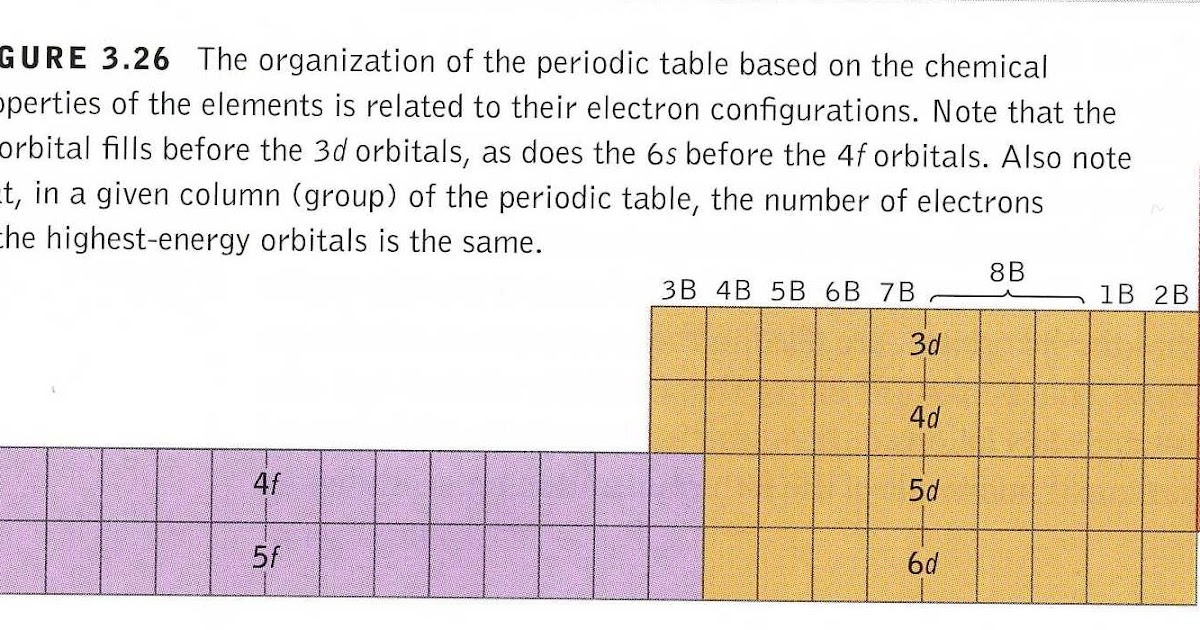
Grayed out electron numbers indicate subshells that are filled to their maximum. So this means we will be filling everything up to the 5p level and then partially fill the.
1S 2S 2P 3S 3P Periodic Table. Which element has the electron configuration 1s^2 2s^2 2p^6 3s^2 3p^2? The first sublevel filled will be the 1s sublevel, then the 2s sublevel, the 2p sublevel, the 3s, 3p, 4s, 3d, and so on.
 Ask the Science Posse "The Rare Earth Question What do From scienceposse.blogspot.com
Ask the Science Posse "The Rare Earth Question What do From scienceposse.blogspot.com
For example, 3s orbital has lower energy than 3p orbitals which again lower energy than the 3d level. Similarly, 3s, 3p and 3d increase energy in that order, and so on. The next six electrons will go in the 2p orbital.
Ask the Science Posse "The Rare Earth Question What do
V __ __ 1s 22s 2p 63s 3p 4s 3d3 4. 1s < 2s < 2p < 3s < 3p < 4s < 3d <. 119 rows 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6 7s 2. The modern periodic table classification like s, p, d, and f block elements is based on properties and general electron or electronic configuration of elements.
 Source: scienceposse.blogspot.com
Source: scienceposse.blogspot.com
Ca 1s 22s 2p 63s 3p 4s You can now fill in the periodic table, starting with the shells with the lowest energy (closest to the nucleus). Therefore the sulfur electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 4. The number of elements you have to move to get to that noble gas is the.





