
Representing moles of ethylene glycol as x we can write: 1 moles/liter = 0.001 moles/millilitre using the online calculator for metric conversions.
15 Ml Of Water To Moles. You need the concentration of the solution to do this. Molarity = 0.15 moles of kmno 4 /0.75 l of solution.
 PPT Crystals PowerPoint Presentation, free download ID From slideserve.com
PPT Crystals PowerPoint Presentation, free download ID From slideserve.com
Molarity = moles solute/liter solution. How many moles of hcl are in 22.15 ml of 4.55 m hcl after it has been diluted with 18.3 ml of water? How many grams are in 10 moles of h2o?
PPT Crystals PowerPoint Presentation, free download ID
Five moles of water whould have a mass of 90.05 g. Mw for hydrogen 1.00 g/ 1 mole; How many moles of water are formed when 15 ml of 0.40 m lioh is added to 25 ml of 0.60 m h2so4? How many moles of water are present in 15.00 ml of water that has a density of 0.9956 g/ ml.
 Source: e-eduanswers.com
Source: e-eduanswers.com
We assume you are converting between grams water and mole. (b) how many moles of naoh were required to neutralize the amount of khp in part (a)? Some students prefer to use a molarity triangle. The si base unit for amount of substance is the mole. From the periodic table we see the atomic weight of hydrogen is 1.0079 and.
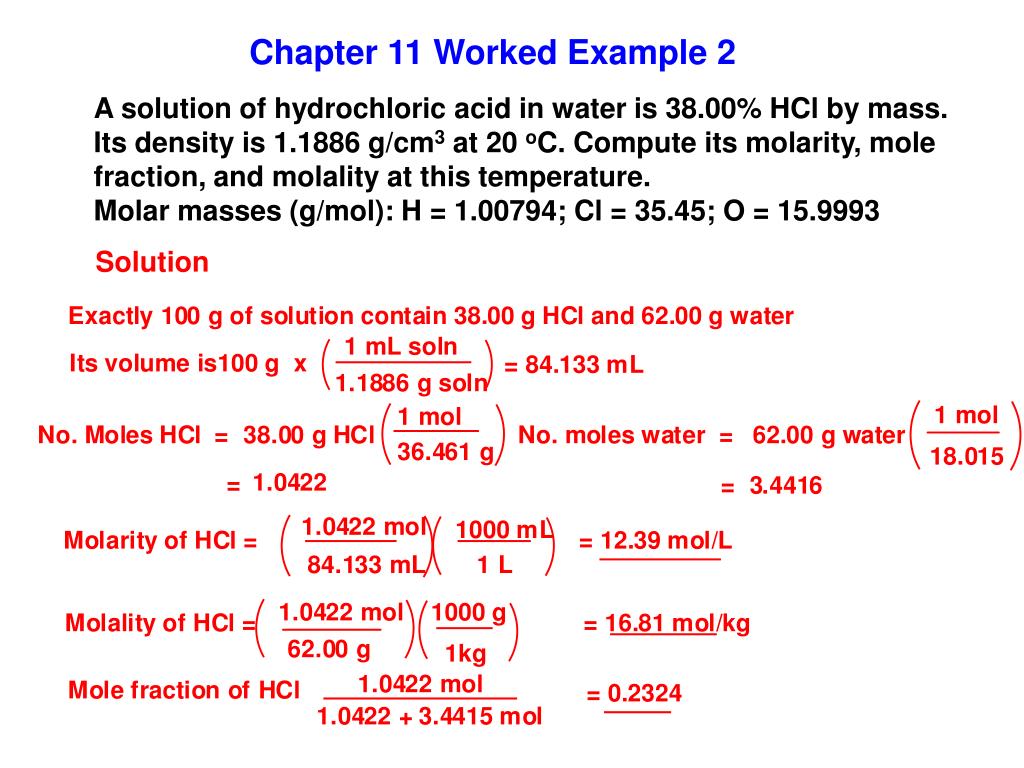 Source: slideserve.com
Source: slideserve.com
One molecule of water (h2o) would weigh 18.02 amu (2×1.00797 amu for h + 15.9994 amu for o), and a mole of water molecules would weigh 18.02 grams. We assume you are converting between grams water and mole. Molecular weight of water or mol. How many moles of hcl are in 22.15 ml of 4.55 m hcl after it has.
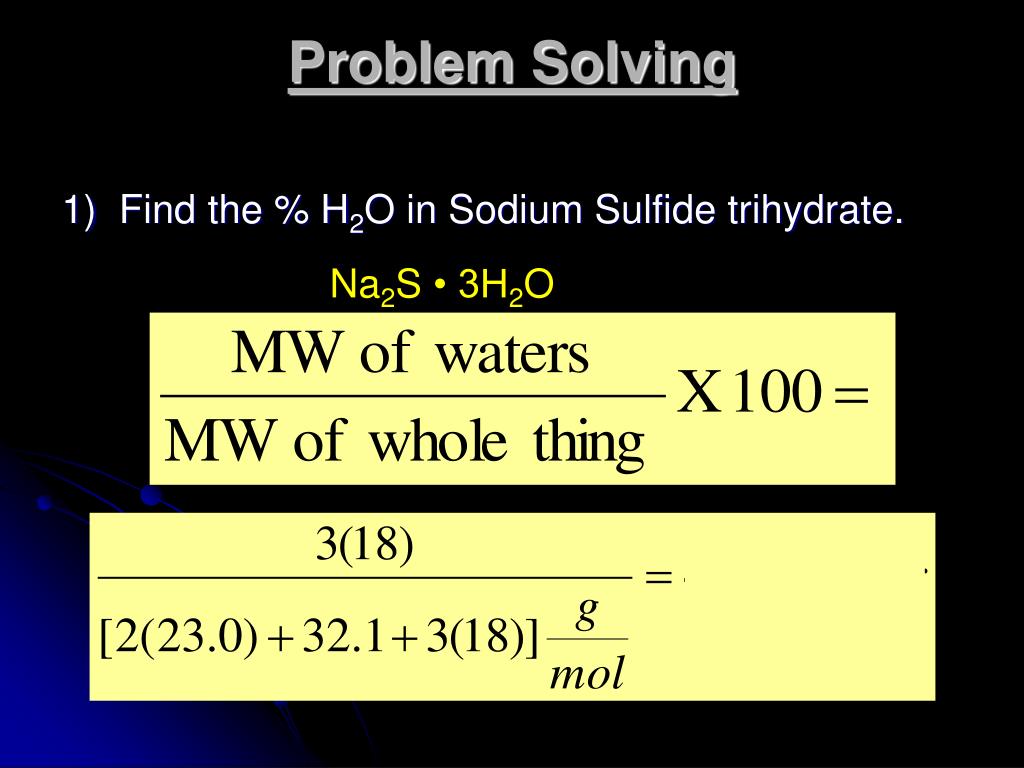 Source: slideserve.com
Source: slideserve.com
He found out that it requires 20. * 1000 ml of 2.1m na2co3 contains= 2.1 moles. Ml of naoh to reach the endpoint of the titration. A formula unit in chemistry is the empirical formula of any ionic or covalent network solid compound used as an independent entity for stoichiometric calculations. This data gives a relationship between amount of solute.
 Source: mathematics-chemistry.blogspot.com
Source: mathematics-chemistry.blogspot.com
Mw for hydrogen 1.00 g/ 1 mole; Let�s calculate this:water density is: In enough water to make 100.0 ml of solution? A formula unit in chemistry is the empirical formula of any ionic or covalent network solid compound used as an independent entity for stoichiometric calculations. Calculate the molarity of the sr(oh) 2.





